Small-cap biopharma Vanda Pharmaceuticals Inc. VNDA has withstood the market-wide sell-off witnessed in early August, thanks to the second-quarter report the company issued July 31.
The earnings report revealed a surprise profit and 25% jump in revenue. The company also reiterated its net product sales guidance for the fiscal year 2019.
Notwithstanding the strong quarterly report and subsequent bounce, Vanda shares are still down about 43% year-to-date. In the same period, the iShares Nasdaq Biotechnology ETF IBB has advanced 9.9%.
Vanda, which commenced operations in early 2003, was founded by Mihael Polymeropoulos, a former Novartis AG NVS executive.
The company went public in 2006 through an initial public offering of 5.75 million shares at a price of $10. After trading roughly sideways in a range from late 2016 to early May 2018, Vanda began an uptrend following the release of its fiscal year 2018 first-quarter results on May 2 of that year.
The stock was in a broader uptrend until January 2019 before reversing course and undergoing a pullback that took it to its 52-week low of $11.83 on July 25. 
Source: Y Charts
The Product Stable
Vanda has two commercial products: Hetlioz and Fanapt.
Hetlioz 20mg capsules, chemically tasimelteon, were approved by the FDA in January 2014 for the treatment of non-24-hour sleep-wake disorder, a serious, rare and chronic circadian rhythm disorder characterized by the inability to synchronize the master body clock with the 24-hour day-night cycle.
It was commercially launched in the U.S. in April 2014. The European Commission granted marketing clearance for Hetlioz in July 2015 as a treatment option for the indication in totally blind adults.
For fiscal year 2018, Hetlioz fetched Vanda net product sales of $115.835 million, or roughly 60% of the company's total revenue, with year-over-year growth of 29%.
Fanapt, chemically iloperidone, is indicated for schizophrenia in adults.
The oral formulation of the drug was approved by the FDA in May 2009 and commercially launched in the U.S. by Novartis in January 2010.
Novartis transferred back all U.S. and Canadian commercial rights to Vanda in December 2014. Fanapt rang up annual sales of $77.28 million in 2018 and posted modest 3% year-over-year growth.
Vanda's Pipeline
Hetlioz is being evaluated for jet lag disorder, Smith-Magenis Syndrome and pediatric non-24-hour sleep-wake disorder.
The company is also assessing additional clinical opportunities for Hetlioz in treating delayed sleep phase disorder, and for sleep disorders in patients with neurodevelopmental disorders.
Vanda is enrolling subjects in a pharmacokinetic study for a long-acting injectable formulation of Fanapt.
Clinical opportunities for Fanapt in biopolar depression are also being assessed.
VLY-686, or tradipitant, is being studied in late-stage clinical trials for treating chronic pruritus in atopic dermatitis as well as for gastroparesis. A Phase 3 trial for gastroparesis is underway, with randomization of patients in the trial likely to occur in the third quarter. Tradipitant is also being studied in a Phase 2 trial for motion sickness.
VTR-297 is in a Phase 1 trial for hematologic malignancies.
VQW-765 is a Phase 2 alpha-7 nicotinic acetylcholine receptor partial agonist.
See also: Biotech Stock On The Radar: Evoke Pharma Sets Date With FDA
Upcoming Catalysts
- PDUFA date for the Hetlioz sNDA in jet lag disorder: Aug. 16.
- The sNDA for Hetlioz in Smith-Magenis Syndrome: third quarter of 2019.
- Initiation of a Phase 2 study of Hetlioz in delayed sleep phase disorder in patients with a mutation in the CRY1 gene: third quarter of 2019.
- Initiation of a Phase 3 program for tradipitant for motion sickness: 2019.
- Randomized study of Fanapt in bipolar disorder: planned to start this year.
- Regulatory filing for marketing authorization for tradipitant in motion sickness: 2020.
- Initiation of a second Phase 3 study of tradipitant for atopic dermatitis: first quarter of 2020.
- Results from a Phase 3 study of tradipitant in atopic dermatitis: first half of 2020.
Vanda received a communication from the FDA July 22 regarding deficiencies the agency identified in the Hetlioz sNDA that preclude labeling and post-marketing commitments at this time. The agency said the communication does not reflect a final decision on the information under review.
The stock reacted with a 4.5% move to the downside in that session. The company said at the time that it expects to receive additional communication from the FDA regarding the deficiencies — and hopes to work expeditiously to resolve them.
The Financials
Vanda has been consistently seeing strong top-line growth due to its product momentum. 
Revenue for the three months ended June 30 increased from $47.35 million in 2018 to $59.06 million in 2019, a 25% jump.
The net income per share trebled to 21 cents, thanks to top-line growth, reining in of operating expenses and higher other income.
Following its second-quarter results, Vanda reiterated its 2019 revenue guidance of $215 million to $225 million, with Hetlioz sales estimated at $137 million to $143 million and Fanapt at $78 million to $82 million.
Analysts, on average, estimate full-year earnings of 34 cents per share on revenue of $223.5 million.
Vanda's cash and cash equivalents and marketable securities totaled $292.7 million as of June 30, 2019, up from $257.36 million at the end of December 2018. Based on the cash burn rate in 2018 and the liquid cash available at the end of the period, Vanda has cash reserves that will last 39 months.
Expert Takes On Vanda
The average analyst recommendation for Vanda is a Buy and the average price target $27.83, according to the Yahoo Finance database.
The average price target represents upside potential of about 85%.
Citi recently upgraded Vanda from Neutral to Buy, citing valuation. The firm has a price target of $19, representing upside of about 27% from current levels.
Vanda has long-term resistance in the $18-$20 region. Once the stock moved below this area to the downside in mid-April, it began a downtrend that was broken following the release of the company's second-quarter print. 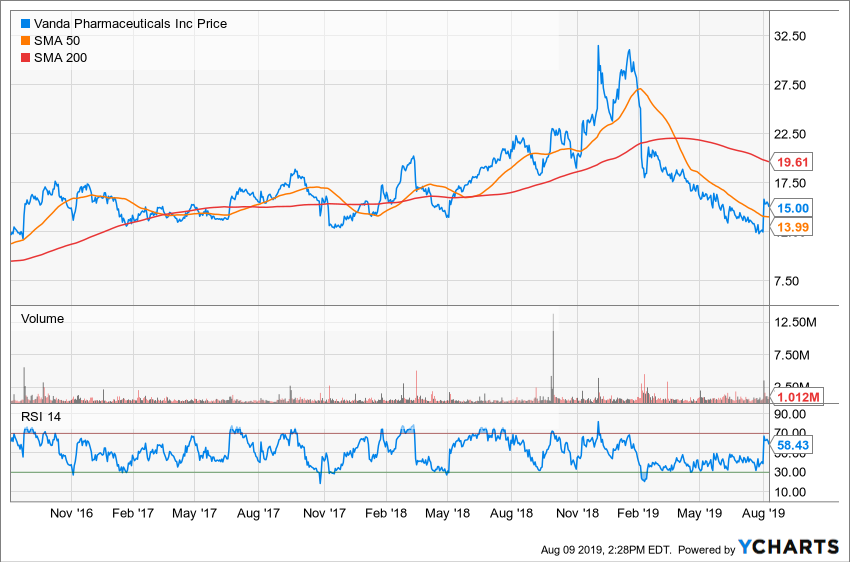
Source: Y Charts
A positive verdict on the binary event could see the stock test this resistance level.
With any potential downside, the $12-$13 area could offer support to the stock.
The simple moving average flashes a red signal, as the shorter-term 50-day SMA is found below the longer-term 200-day SMA. The 14-day RSI suggests the stock is in neutral territory.
Related Link: Biotech Stock On The Radar: Veru, A Catalyst-Rich Biopharma
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Comments
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.