I’ve been telling investors for over a year now that when the first psychedelics pure play goes public, it’s going to crush it.
And that’s exactly what happened.
This week, MindMed MMEDF went public on the NEO exchange, and also in the U.S. with an OTC listing, trading under the symbol MMEDF.
At the time of this writing, here’s how both have performed …
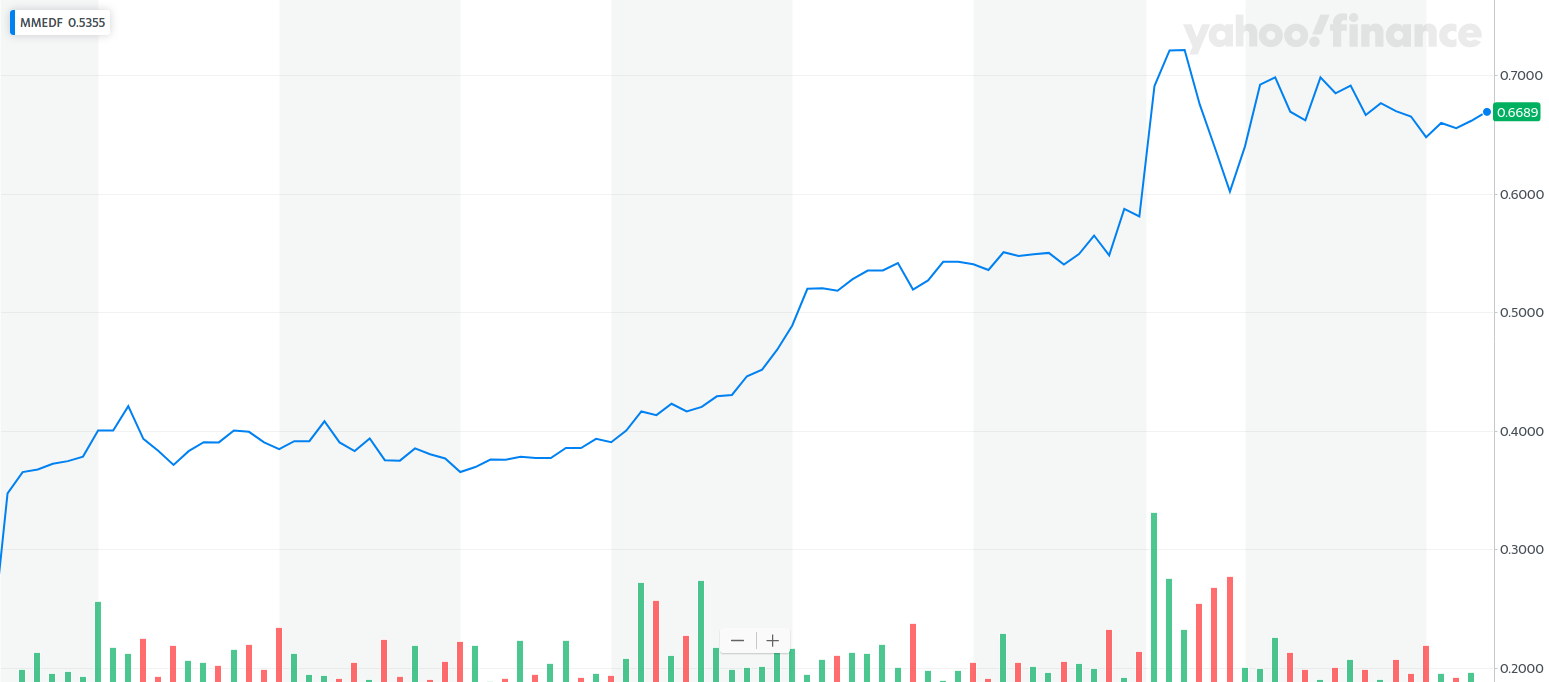
And
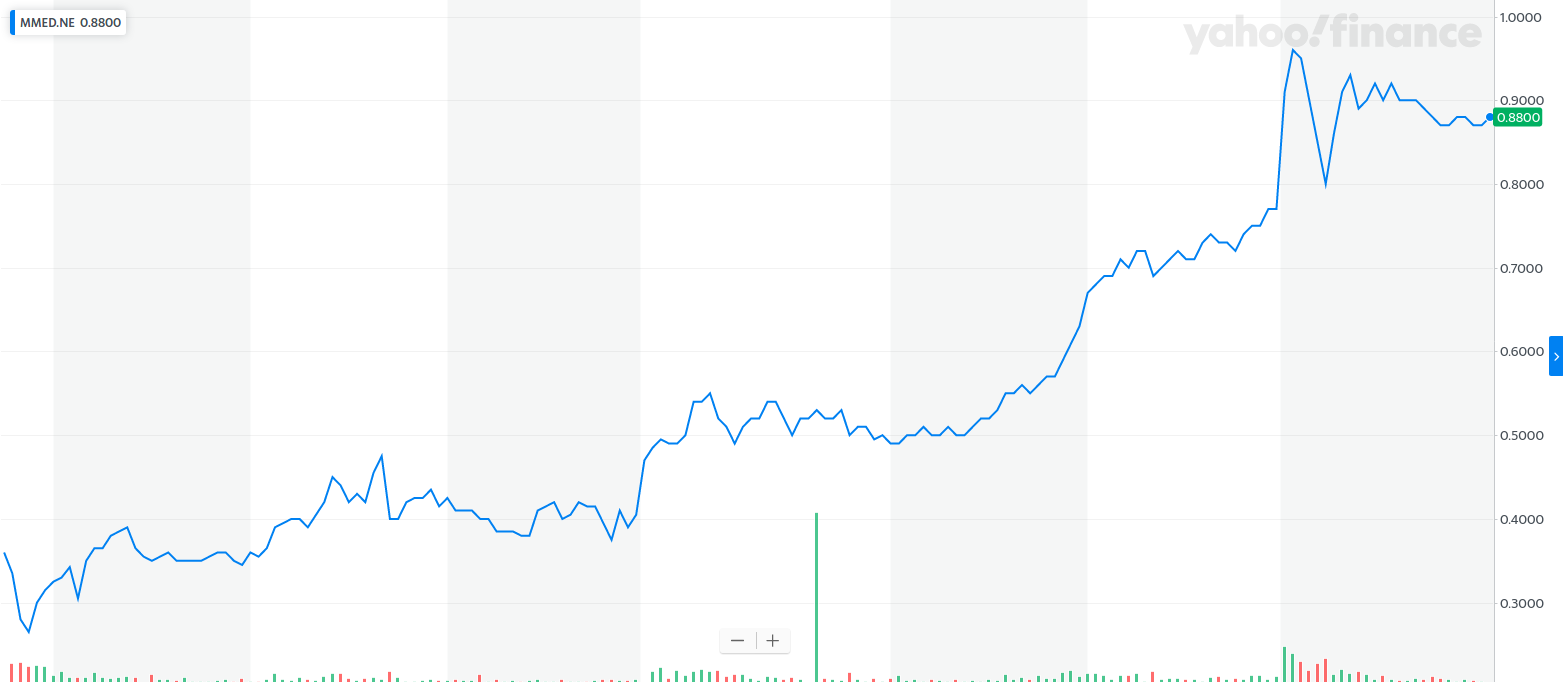
I have little doubt that those who get an early piece of MindMed will be sitting on a quadruple-digit gain within three years.
Make no mistake: The psychedelics industry is going to take the market by storm. And because pickings are slim right now, it’s pretty much like shooting fish in a barrel.
Of course, MindMed is a serious contender.
If you’re unfamiliar, MindMed is building an IP portfolio and undertaking clinical trials of medicines based on psychedelics. It will also grow its pipeline through acquisitions, joint-ventures, and collaborative development programs.
What makes MindMed particularly interesting, however, is its focus …
MindMed is developing two categories of medicines based on psychedelic substances: Hallucinogenic therapies and non-hallucinogenic medicines. It’s the second one that makes this particularly attractive.
You see, the primary obstacle to getting these medicines into the marketplace is strict regulatory control.
Regarding hallucinogenic therapies, direct supervision by a therapist or doctor is required, and only in a regulated clinic can these substances be used.
But for non-hallucinogenic psychedelics, with FDA approval, all that’s needed is a doctor’s prescription and a pharmacy pickup.
So when we look at the psychedelics space, the non-hallucinogenic therapies are being seen as the low-hanging fruit. And MindMed is working with two non-hallucinogenic psychedelics right now.
See Also: Psychedelic-Enhanced Psychotherapy Clinic Opens In Toronto
The first is called 18-MC, which is derived from Ibogaine. It’s currently preparing Phase II FDA clinical trials for opioid use disorder.
Today, 11 million Americans are misusing opioids. MindMed is developing an anti-addictive molecule based on Ibogaine that treats addiction as a brain disease. Not only is something like this a game-changer, but it’s something that can be done in coordination with a major pharmaceutical company, or just sold outright to one.
Unlike the way medical cannabis came onto the market, psychedelics do have to work directly with the FDA. The downside to this is the onerous FDA process. The upside - which is major - is that Big Pharma can play in this space, because the FDA is involved, and these medications, once approved, can be sold throughout pharmacies all across the country. This should not be trivialized at all.
Now the second non-hallucinogenic psychedelic that MindMed is working on right now is micro-dosed LSD to treat ADHD. The company is preparing for Phase II clinical trials on this one right now.
Worth noting is that there’s a growing trend in silicon valley to take small amounts of LSD with no hallucination effect to increase focus and creativity. MindMed is undertaking clinical trials to prove it actually works. The anecdotal evidence suggests that it does. And I personally know two people who have been doing this with excellent results.
Of course, anecdotal evidence isn’t enough. But make no mistake: With FDA approval, the opportunity here is huge.
Take Spravato, for instance.
Spravato represents the first psychedelic treatment approved by the FDA for a psychiatric condition. It’s a non-hallucinogenic psychedelic treatment, and by 2024, its expected to deliver sales in excess of $1.7 billion. That’s billion, with a “B.” From one drug.
The upside of FDA-approved psychedelics is undeniable. And while I do see plenty of opportunity for hallucinogenic psychedelics to successfully pass FDA trials, too, it’ll likely be the non-hallucinogenic ones that will get to market first.
And what makes this particularly interesting is that we’re now seeing some indication that psychedelic medicines could be far superior than current FDA-approved medications on the market designed to treat addiction.
The thing is, when it comes to treatment for addiction, current FDA-approved treatments don’t actually address the mechanism of addiction. They’re substitution therapies, like nicotine patches, for instance, and those have addictive properties as well, and with low efficacy and high rates of relapse.
When it comes to opioid addiction, this is a huge problem.
The opioid crisis in America costs the economy $500 billion a year, with nearly 2 million people suffering from opioid use disorder. Yet when we look at treatments, success rates are tragically low.
Take Suboxone, for instance, which is used to “treat” opioid addiction.
The failure rate of Suboxone is about 90% after a 12-week treatment. This is hardly an effective treatment. And quite frankly, is just substituting one addiction for another.
Meanwhile, a study using Ibogaine (this is what MindMed’s 18-MC is derived from), has demonstrated that 50% of opioid addicts are opiate-free just 4 weeks after withdrawal.
This is another very big deal, and the FDA is taking notice.
The FDA has already granted three “Breakthrough Therapy” designations for other psychedelic treatments, with the most recent being given to the Usona Institute, which is developing a psilocybin-based treatment for major depressive disorder.
If you’re unfamiliar, Breakthrough Therapy designation is a process designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s).
It is clear that the FDA is open to offering Breakthrough Therapy Designations for psychedelics. And with the opioid crisis still very much alive and well in America, the FDA is eager to get something new out on the market.
18-MC could be a very real contender here.
And the investment community - at least those who regularly write 7-figure checks - are taking notice, too.
Prior to its IPO, a lot of capital was flooded into MindMed, particularly from some pretty high-profile investors, including ...
- Bruce Linton - former CEO of Canopy Growth Corporation GGC
- Kevin O’Leary - Super wealthy entrepreneur and part of the hit show Shark Tank
- James Bailey - Partner of private equity firm Bail Capital
More than $6 million was raised just through those three, and $24M was raised prior to the IPO.
MindMed clearly has a first-mover advantage, and investors would be wise to jump on its coattails for a long and profitable ride.
Photo by Javier Hasse. Charts from Yahoo Finance.
Disclosure: Jeff Siegel is long MindMed.
The preceding article is from one of our external contributors. It does not represent the opinion of Benzinga and has not been edited.
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Comments
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.
Click on the image for more info.
Cannabis rescheduling seems to be right around the corner
Want to understand what this means for the future of the industry?
Hear directly for top executives, investors and policymakers at the Benzinga Cannabis Capital Conference, coming to Chicago this Oct. 8-9.
Get your tickets now before prices surge by following this link.