MELBOURNE, Australia, Dec. 16, 2019 /PRNewswire/ -- Antisense Therapeutics ("ANP" or the "Company") is pleased to advise that the data from all nine participants having completed their 24 weeks of dosing in the Phase II clinical trial of ANP's immunomodulatory therapy, ATL1102 for Duchenne Muscular Dystrophy (DMD) has affirmed the drug's excellent safety profile and positive drug effects on disease progression endpoints at the low dose tested and in turn the Company's plans to advance ATL1102 into a potentially pivotal Phase IIb clinical trial.
William Goolsbee, non-executive director of ANP, Chairman of the ATL1102 for DMD Scientific Advisory Board and former-Chairman of Sarepta Therapeutics said: "Seeing the efficacy signals of this study, conducted with a low dose in a small number of boys over a relatively short time period, is both gratifying and immensely encouraging. DMD is a devastating disease where only a small handful of drugs have shown indications of efficacy so early in development. In the context of DMD, we now look to have a drug. We have a great task ahead of us as we move ATL1102 into deeper study with the goal of providing treatment options to all, not just some, of the boys with DMD."
ATL1102 is an inhibitor of CD49d expression on certain immune cells (e.g. T lymphocytes). It has been reported (Pinto-Mariz et al 2015) that DMD patients who have a greater number of T cells with high levels of CD49d have more severe and rapid disease progression. ATL1102 is the only drug in clinical development for DMD targeting CD49d and one of a very limited number of treatments being tested in wheelchair bound DMD boys who are at a more advanced stage of the disease.
The primary objective of the ATL1102 trial is to assess the safety and tolerability of 25 mg of ATL1102 administered once weekly (subcutaneous injection) for 24 weeks in non-ambulatory DMD participants.
ATL1102 appears to be generally safe and well tolerated in non-ambulant boys with DMD. No Serious Adverse Events have been reported and there have been no safety concerns expressed by the Data Safety Monitoring Board. There were no participant withdrawals from the study. The most commonly reported adverse events have related to the subcutaneous administration of the drug, mainly injection site erythema and skin discoloration.
The trial also assessed drug activity and efficacy including measuring the effects on immune cells numbers in the blood and measuring functional capacity in participants as evaluated via Performance of Upper Limb Test (PUL2.0) and upper limb strength via MyoGrip and MyoPinch assessments. These are standard tests for assessing disease improvement, stabilization or progression in non-ambulant DMD patients.
Notably, the immune cell data has shown a consistency in the mean reductions in the number of lymphocytes and types of lymphocytes (i.e. CD3, CD4, CD8 and those expressing CD49d) measured from baseline to end of dosing at week 24 with a return to around starting levels post dosing at week 28 (refer table 1). The mean number of CD3+CD49d+ T cells (CD4+CD49d and CD8+CD49d+ cells) at week 24 is statistically significantly lower vs week 28 (p=0.030 paired T test). This data is supportive of the drug's positive effects on modulating CD49d+ T cells in the blood. A subset of these T cells express high levels of CD49d, and are present in higher numbers in non-ambulant boys as reported by Pinto-Mariz et al.
In commenting on the above observations regarding lymphocyte changes, Dr Pinto-Mariz from the Institute of Pediatrics, Federal University and the Laboratory on Thymus Research, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil said "I am pleased to see the ATL1102 drug targeting CD49d in DMD patient trials following on from our work and publications on the importance of the CD49d expressing cells in DMD. I am very happy that ATL1102 reduced T cells expressing CD49d which should enable the assessment of our hypothesis that the number of T cells expressing high levels of CD49d is a progression and severity marker in non-ambulant DMD patients".
With regard to the endpoints that assess the drug's effect on disease progression, the PUL2.0 data as reported in Table 2 shows that 7 of the 9 participants demonstrated either increases or no change in their PUL2.0 scores from baseline, suggestive of an overall improvement with a positive mean change of 0.9 in this key parameter.
MyoGrip and MyoPinch assessments using the Myoset system are also reported in Table 2 with the data continuing to show a distinct improvement in muscle strength based on the observed mean changes from baseline compared to the loss of muscle strength reported in the Ricotti et al 2016 publication in a similar non ambulant patient population on corticosteroids. The Forrest plot reported as Graph 1, shows the improvements in grip and pinch strength in patients on ATL1102 compared to the loss of muscle strength reported in the Ricotti et al 2016 to be statistically significant.
Respiratory parameters (FVC and PEF) were also measured. In our experts' view the variability in this data appears consistent with the inherent variability associated with these measurements making it difficult to draw any conclusions on this parameter, which generally requires substantially more subjects be treated over a longer time frame (12 months and longer) to produce interpretable changes.
The Phase II trial follow up period is continuing with the last two patients in the monitoring phase. The Last Participant Last Visit for the study will be in the beginning of January 2020 with the final study report to be prepared following trial database lock expected 1Q'20.
The Company has been consulting with internationally recognized DMD experts in regard to analyzing the trial data to evaluate the response to therapy within the ATL1102 trial. With reference to the well regarded analysis of the loss of function in non-ambulant patients (most on standard corticosteroid therapy) in publications by Pane et al 2018 and Ricotti et al 2019 and what the authors viewed as a clinically meaningful change on PUL and Myoset measurement parameters, the ATL1102 study appears to show 3 patients (2, 4 and 8) as having improved by a clinically meaningful amount on both PUL and Grip strength. Another patient (1) had a similar clinical improvement on PUL2.0 alone and further 3 (3, 9, and 10) stabilized on this parameter.
Professor Thomas Voit MD, Director, NIHR GOSH Biomedical Research Centre, UK who is an author on the Pinto-Mariz et al 2015 and Ricotti et al 2019 publications had this to say about the trial results and the efficacy being observed in this Phase IIa trial of ATL1102 - "Disease stabilisation or indeed improvement in functional scores in non-ambulant DMD boys is almost unheard of and a very encouraging result. This is even more meaningful as these results have been obtained using different independent measures and over a relatively short trial time of 24 weeks. These results also advise on endpoint choice for a fully powered placebo-controlled registration-enabling study."
The results continue to be highly supportive of the Company's plans for a Phase IIb clinical trial of ATL1102 in DMD. Over the recent months Scientific Advice meetings have been held with three European regulatory authorities with a focus on the Phase IIb trial design, dose escalation plans, applicability of the study end-points and the study duration. There was general acceptance by the agencies at the meetings on the proposed trial efficacy endpoints (PUL2.0, Myoset), safety monitoring plan, dosing duration (12 months) and the use of higher doses. Encouraging clarification was provided by the agencies that this could be a path to an early regulatory approval on positive Phase IIb results. The next step will be to follow up the development plan with the European Medicines Agency and subsequent to the finalisation of the results from the current Phase II trial, engage with the Food and Drug Administration on development plans for the US.
Mark Diamond, CEO of Antisense Therapeutics said: "We had high expectations for ATL1102 in DMD, and this first study has certainly exceeded them with respect to its efficacy signal at the lower dose tested. The next stage of development will be in translating what we have learned into optimizing clinical benefit for the non-ambulatory boys who comprise ~50% of the total DMD population and who have no effective treatment options. The Company is moving forward with all deliberate speed to advance ATL1102 through the clinic, with specific view to a blinded controlled study in the EU which, based upon recent and on-going guidance, may lead directly to early approval."
For more information please contact: | |
Antisense Therapeutics | Investment Enquiries |
Mark Diamond | Gennadi Koutchin |
Managing Director | XEC Partners |
+61 (0)3 9827 8999 | |
1300 932 037 | |
About Antisense Therapeutics Limited ANP is an Australian publicly listed biopharmaceutical company, developing and commercialising antisense pharmaceuticals for large unmet markets. The products are in-licensed from Ionis Pharmaceuticals Inc. IONS, world leaders in antisense drug development and commercialisation. ATL1102 (injection) has successfully completed a Phase II efficacy and safety trial, significantly reducing the number of brain lesions in patients with relapsing-remitting multiple sclerosis (RRMS). ATL1103 drug designed to block GHr production successfully reduced blood IGF-I levels in Phase II clinical trials in patients with the growth disorder acromegaly. The Company is conducting a Phase II clinical trial of ATL1102 in DMD patients at the Royal Childrens Hospital, Melbourne.
About ATL1102 ATL1102 is an antisense inhibitor of CD49d, a subunit of VLA-4 (Very Late Antigen-4). Antisense inhibition of VLA-4 expression has demonstrated activity in a number of animal models of inflammatory disease including asthma and MS with the MS animal data having been published in a peer reviewed scientific journal. ATL1102 was shown to be highly effective in reducing MS lesions in a Phase IIa clinical trial in RR-MS patients. The ATL1102 Phase IIa clinical data has been published in the medical Journal Neurology (Limmroth, V. et al Neurology, 2014; 83(20): 1780-1788).
About ATL1102 DMD Trial The Company is undertaking a clinical trial of ATL1102 in patients with Duchenne Muscular Dystrophy. The open label six-month dosing trial of ATL1102 in nine non-ambulant patients with DMD aged between 10 and 18 years is being conducted at the neuromuscular centre of the Royal Children's Hospital (RCH) which operates the largest clinic in the southern hemisphere treating children with DMD. The primary endpoints of the trial relate to the safety and tolerability of ATL1102. The efficacy of ATL1102 will also be assessed in terms of its effects on disease processes and progression (e.g. the upper limb strength and function of the boys). Further details on the trial are available here on the Australia and New Zealand Clinical Trials Registry.
About DMD Duchenne Muscular Dystrophy (DMD) is an X-linked disease that affects 1 in 3600 to 6000 live male births (Bushby et al, 2010). DMD occurs as a result of mutations in the dystrophin gene which causes a substantial reduction in or absence of the dystrophin protein. Children with DMD have dystrophin deficient muscles and are susceptible to contraction induced injury to muscle that triggers the immune system which exacerbates muscle damage as summarized in a publication co-authored by the Director of the FDA CDER (Rosenberg et al, 2015). Ongoing deterioration in muscle strength affects lower limbs leading to impaired mobility, and also affects upper limbs, leading to further loss of function and self-care ability. The need for wheelchair use can occur in early teenage years for patients on corticosteroids with a mean age of 13, with respiratory, cardiac, cognitive dysfunction also emerging. Patients with a greater number of immune T cells expressing high levels of CD49d have more severe and progressive disease and are wheelchair bound by the age of 10 despite being on corticosteroid treatment (Pinto Mariz et al, 2015). With no intervention, the mean age of life is approximately 19 years. The management of the inflammation associated with DMD is currently addressed via the use of corticosteroids, however they are acknowledged as providing insufficient efficacy and are associated with significant side effects. As a consequence, there is an acknowledged high need for new therapeutic approaches for the treatment of inflammation associated with DMD.
Rosenberg AS, Puig M, Nagaraju K, et al. Immune-mediated pathology in Duchenne muscular dystrophy. Sci Transl Med 2015, 7: 299rv4.
Busby et al for the DMD Care Consideration Working Group/ Diagnosis and management of Duchenne muscular dystrophy, part 1 Lancet Neurol. 2010 Jan;9(1):77-93 and part 2 Lancet Neurol. 2010 Feb;9(2):177-89 .
Pinto-Mariz F, Carvalho LR, Araújo AQC, et al. CD49d is a disease progression biomarker and a potential target for immunotherapy in Duchenne muscular dystrophy. Skeletal Muscle 2015, 5: 45-55.
Table 1:
White blood cell type (X109 cells per litre) | Mean # and Change from | Median % change from | |||
Baseline | 24 weeks | 28 | 24 weeks | 28 | |
Lymphocytes (mostly CD3+ T cells) | 3.68 | -0.28 | +0.19 | -4.22% | +11.81% |
CD3+ T cells | 2.93 | -0.18 | +0.25 | 0.86% | +17.11% |
CD3+ CD49d+ T cells (CD4+CD49d+ and CD8+CD49d+ cells) | 2.44 | -0.28 | +0.11* | -9.78% | +9.93% |
CD4+ T cells | 1.57 | -0.15 | +0.11 | -1.12% | +16.50 |
CD4+ CD49d+ T cells | 1.20 | -0.19 | +0.01 | -16.7% | +1.73 |
CD4+ CD49d++ T cells | 0.24 | -0.01 | +0.01 | -11.1% | +7.58 |
CD8+ T cells | 1.22 | -0.02 | +0.14 | -2.62% | +17.99 |
CD8+ CD49d+T cells | 1.17 | -0.05 | +0.11 | -5.79% | +13.37 |
CD8+ CD49d++ T cells (5 of 9 patients had these cells at baseline) | - | - | - | -6.17% | +14.12 |
The Lymphocyte mean # of cells at week 24 (at the end of dosing) is trending significantly lower vs week 28 (p= 0.051 paired T test)
The CD3, CD4, CD8, CD4+CD49d+ and CD8+CD49d+ mean # of cells at week 24 are similarly trending lower vs week 28 (p= from 0.056 to 0.073)
*The mean # of CD3+CD49d+ T cells (=CD4+CD49d+ and CD8+CD49d+cells) at week 24 is statistically significantly lower vs week 28 (p= 0.030 paired T test)
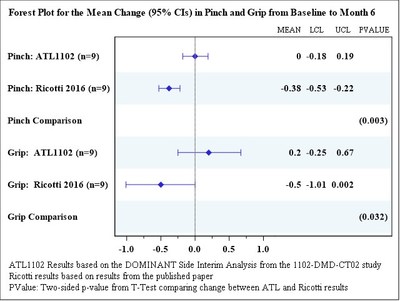
Table 2:
Change from Baseline to Week 24 | |||||||
Patient No. | PUL 2.0 | MyoGrip (dom) (Kg) | MyoGrip (% Pred) | MyoPinch (Kg) | MyoPinch (% Pred) | % Predicted | % Predicted |
01-001 | +2 | -0.63 | -4.49 | 0.03 | -0.62 | -3.20 | 6.30 |
01-002 | +2 | 0.22 | 0.49 | -0.02 | -0.29 | -14.8 | -17.3 |
01-003 | 0 | 0.68 | 1.02 | -0.40 | -6.59 | -9.10 | 8.70 |
01-004 | +2 | 1.09 | 1.01 | 0.37 | 2.99 | 0.80 | 7.20 |
01-006 | -3 | -0.27 | -0.60 | 0.07 | 0.94 | -6.50 | 6.90 |
01-008 | +7 | 1.00 | 1.11 | 0.30 | 2.77 | -7.70 | -18.2 |
10-009 | 0 | -0.33 | -3.75 | -0.22 | -4.97 | -9.10 | -4.30 |
01-010 | 0 | 0.05 | 0.11 | 0.06 | 0.72 | -0.40 | 9.20 |
01-011 | -2 | 0.11 | -1.31 | -0.18 | -3.63 | -1.10 | 2.00 |
Mean Change (95% CI): | 0.9 (-1.33, 3.11) | 0.2 (-0.25, 0.67) | -0.7 (-2.33, 0.90) | 0.0 (-0.18, 0.19) | -1.0 (-3.56, 1.63) | -5.68 (-9.60,-1.76) | 0.06 (-8.33, 8.44) |
SOURCE Antisense Therapeutics Limited
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.