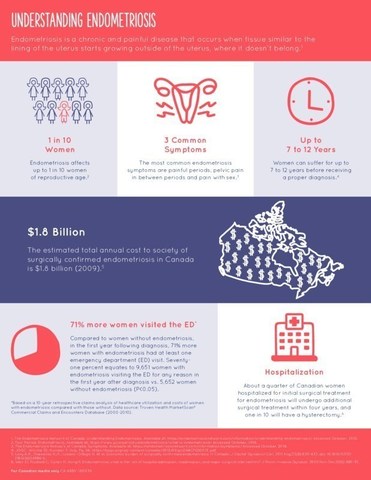
- Endometriosis affects up to one in 10 women of reproductive age in Canada.1
- 7 out of 10 women being managed for endometriosis have unresolved pain throughout the month.2
MONTREAL, May 21, 2019 /CNW/ - AbbVie ABBV, a global, research and development-driven biopharmaceutical company, in cooperation with Neurocrine Biosciences, Inc. NBIX, announced that ORILISSA™ (elagolix) 200 mg twice daily is now available. ORILISSA is the first and only oral gonadotropin-releasing hormone receptor (GnRHr) antagonist, for the treatment of moderate to severe pain associated with endometriosis.3
"Endometriosis is a misunderstood and often mismanaged disease. It has been estimated that on average, it takes 9-10 years for a woman to receive a diagnosis of endometriosis. This is often because patients themselves and physicians normalize the pain these patients are experiencing. This can have a huge impact on their quality of life, relationships and work productivity. Even diagnosed women who suffer from it tend to normalize their pain and downplay the effects it has on all aspects of their lives," says Dr. Jamie Kroft MD, MSc, FRCSC, Assistant Professor, Minimally Invasive Gynaecologic Surgery, Core Obstetrics & Gynaecology, Sunnybrook Health Sciences Centre. "The women I see in my practice always have a lot of questions, especially around appropriate medical management. I take the time to explain their options so they can make an informed decision. Therefore, the more options that are available, for example ones that are hormone-free and can be customized to my patients' needs, the better I can treat them. Women should not suffer in silence, especially not when there are new treatment advances."
ORILISSA (elagolix) is a novel, orally administered, highly potent, short-acting, selective, non-peptide small molecule GnRHr antagonist that blocks endogenous GnRH signaling by binding competitively to GnRH receptors in the pituitary gland. Administration of ORILISSA results in dose-dependent suppression of luteinizing hormone (LH) and follicle-stimulation hormone (FSH) levels, leading to decreased blood levels of the ovarian sex hormones, estradiol and progesterone. LH and FSH suppression begins within hours of administration and is readily reversible upon discontinuation of ORILISSA.3
"At the Endometriosis Network Canada, we encourage our members to become informed and empowered. Having an accurate diagnosis and getting treatment from an endometriosis expert are important steps in attaining relief from their symptoms. Although endometriosis is currently incurable, there are effective treatments available and in partnership with their healthcare professionals, patients can work on a treatment plan that works for them. Ultimately, we know what people living with this debilitating disease want is to live fulfilling and pain-free lives, where they are able to pursue any endeavour, both professionally and personally," explains Philippa Bridge-Cook, Ph.D., Executive Director of The Endometriosis Network Canada.
Endometriosis causes chronic pelvic pain and is sometimes associated with infertility. It affects up to one in 10 women of reproductive age in Canada.1 Furthermore, 7 out of 10 women being managed for endometriosis have unresolved pain throughout the month. 2
The approval of ORILISSA is supported by data from two replicate studies in the largest endometriosis Phase 3 study program conducted to date, which evaluated nearly 1,700 women with moderate to severe endometriosis pain. Clinical trial data demonstrated ORILISSA significantly reduced the three most common types of endometriosis pain: dysmenorrhea, non-menstrual pelvic pain and dyspareunia. A higher proportion of women treated with ORILISSA 150 mg once daily and 200 mg twice daily were responders for daily menstrual pain and non-menstrual pelvic pain compared to placebo in a dose- dependent manner at month three. Women were defined as responders if they experienced a clinically meaningful reduction in daily menstrual pain and non-menstrual pelvic pain with no increase in analgesic use (nonsteroidal anti-inflammatory drug or opioid) for endometriosis-associated pain.3
Both ORILISSA treatment groups showed statistically significant greater mean decreases from baseline compared to placebo in daily menstrual pain and non-menstrual pelvic pain at month six. Women in the Phase 3 studies also provided a daily self-assessment of their endometriosis pain using a numeric rating scale (NRS) and women taking ORILISSA 150 mg once daily and 200 mg twice daily reported a statistically (p <0.001) significant reduction from baseline in NRS scores compared to placebo at month three. Clinical trial data also demonstrated women taking ORILISSA 200 mg twice daily showed statistically significant greater reduction in pain during sexual intercourse from baseline to month three compared to placebo. The most frequent (≥10%) adverse reactions reported in clinical trials with ORILISSA (elagolix) were hot flush, headache and nausea.3
The recommended duration of use for ORILISSA is up to 12months for the 150 mg once daily dose and up to six months for the 200 mg twice daily dose, as it causes a dose-dependent decrease in bone mineral density (BMD). BMD loss is greater with increasing duration of use and may not be completely reversible after stopping treatment.3
"We are proud to launch the ORILISSA 200 mg strength. With this dose, we are able to offer physicians the unique ability to individualize the care of their patients. Women with endometriosis now have a hormone-free choice that is customizable based on their unique needs," says Stéphane Lassignardie, General Manager of AbbVie Canada. "AbbVie is committed to women living with endometriosis as we strive to fill the unmet medical need by providing a safe and efficacious treatment."
About AbbVie Care
Canadian women prescribed ORILISSA will have the opportunity to be enrolled in AbbVie Care, AbbVie's signature support program. The program is designed to provide a wide range of customized services including reimbursement and financial support, pharmacy services, personalized education and ongoing disease management support throughout their treatment. For more information, please visit www.abbviecare.ca.
About AbbVie
AbbVie is a global, research and development-driven biopharmaceutical company committed to developing innovative advanced therapies for some of the world's most complex and critical conditions. The company's mission is to use its expertise, dedicated people and unique approach to innovation to markedly improve treatments across four primary therapeutic areas: immunology, oncology, virology and neuroscience. In more than 75 countries, AbbVie employees are working every day to advance health solutions for people around the world. For more information about AbbVie, please visit us at www.abbvie.ca and www.abbvie.com. Follow @abbvieCanada and @abbvie on Twitter or view careers on our Facebook or LinkedIn page.
About Neurocrine Biosciences, Inc.
Neurocrine Biosciences, a San Diego based biopharmaceutical company, is focused on developing treatments for neurological and endocrine related disorders. The company discovered, developed and markets INGREZZA® (valbenazine), the first FDA approved product indicated for the treatment of adults with tardive dyskinesia, a movement disorder. Discovered and developed through Phase II clinical trials by Neurocrine, ORILISSA™ (elagolix), the first FDA-approved oral medication for the management of endometriosis with associated moderate to severe pain in over a decade, is marketed by AbbVie as part of a collaboration to develop and commercialize elagolix for women's health. Neurocrine's clinical development programs include opicapone as an adjunctive therapy to levodopa/DOPA decarboxylase inhibitors in Parkinson's disease patients, elagolix for uterine fibroids with AbbVie, valbenazine for the treatment of Tourette syndrome, and NBI-74788 for the treatment of congenital adrenal hyperplasia (CAH). For more information and the latest updates from Neurocrine, please visit www.neurocrine.com.
1 | YourPeriod.ca. https://www.yourperiod.ca/endometriosis/what-is-endometriosis/. Accessed May 2019. |
2 | De Graaff AA, D'Hooghe TM, Dunselman GAJ, Dirksen CD, Hummelshoj L, WERF EndoCost Consortium, Simoens S. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677-2685. |
3 | Orilissa Product Monograph, AbbVie Corporation, October 4, 2018. http://www.abbvie.ca/content/dam/abbviecorp/ca/en/docs/ORILISSA_PM_EN.PDF. Accessed May 2019. |

SOURCE AbbVie
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/May2019/21/c8612.html
© 2024 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.
Trade confidently with insights and alerts from analyst ratings, free reports and breaking news that affects the stocks you care about.